Cl Valence Electrons
- Cl Number Of Valence Electrons
- List Of Valence Electrons For Each Element
- Cl Number Of Valence Electrons
- Cl Element Valence Electrons
Valence electrons by definition are the number of electrons in the valence shell. The atomic number of Chlorine is 17. Hence the configuration of electrons would be 2,8,7. Hence the number of valence electrons in Chlorine is 7. However if you tal. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining five electrons. Therefore the Chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 5.
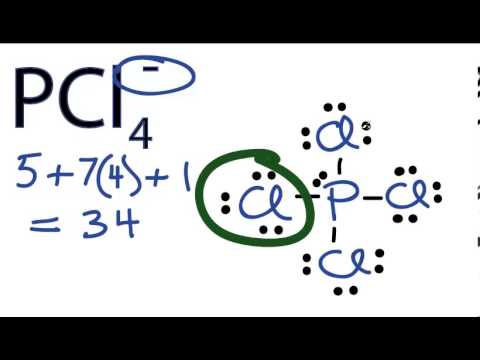
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. When discussing the octet rule, we do not consider d or f electrons. Only the s and p electrons are involved in the octet rule, making it useful for the main group elements (elements not in the transition metal or inner-transition metal blocks); an octet in these atoms corresponds to an electron configurations ending with (s^2p^6).
Introduction
In 1904, Richard Abegg formulated what is now known as Abegg's rule, which states that the difference between the maximum positive and negative valences of an element is frequently eight. This rule was used later in 1916 when Gilbert N. Lewis formulated the 'octet rule' in his cubical atom theory. Atoms will react to get in the most stable state possible. A complete octet is very stable because all orbitals will be full. Atoms with greater stability have less energy, so a reaction that increases the stability of the atoms will release energy in the form of heat or light.
Octet Rule
A stable arrangement is attended when the atom is surrounded by eight electrons. This octet can be made up by own electrons and some electrons which are shared. Thus, an atom continues to form bonds until an octet of electrons is made.
- Normally two electrons pairs up and forms a bond, e.g., (H_2)
- For most atoms there will be a maximum of eight electrons in the valence shell (octet structure), e.g., (CH_4)
Note
The noble gases rarely form compounds. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their configuration. All other elements attempt to gain, lose, or share electrons to achieve a noble gas configuration.
Example 1: NaCl Salt
The formula for table salt is NaCl. It is the result of Na+ ions and Cl- ions bonding together. If sodium metal and chlorine gas mix under the right conditions, they will form salt. The sodium loses an electron, and the chlorine gains that electron. In the process, a great amount of light and heat is released. The resulting salt is mostly unreactive — it is stable. It will not undergo any explosive reactions, unlike the sodium and chlorine that it is made of. Why?
Solution
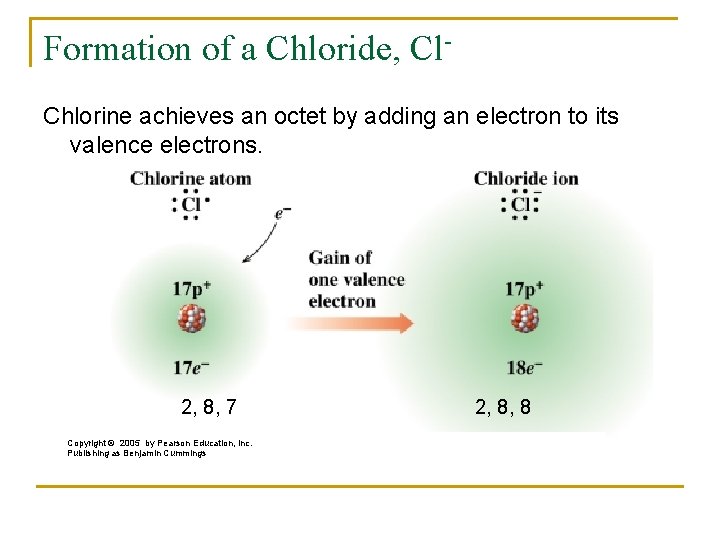
Referring to the octet rule, atoms attempt to get a noble gas electron configuration, which is eight valence electrons. Sodium has one valence electron, so giving it up would result in the same electron configuration as neon. Chlorine has seven valence electrons, so if it takes one it will have eight (an octet). Chlorine has the electron configuration of argon when it gains an electron.
The octet rule could have been satisfied if chlorine gave up all seven of its valence electrons and sodium took them. In that case, both would have the electron configurations of noble gasses, with a full valence shell. However, their charges would be much higher. It would be Na7- and Cl7+, which is much less stable than Na+ and Cl-. Atoms are more stable when they have no charge, or a small charge.
Contributors and Attributions
03
Based on the periodic table the , the atomic number (Z) of chlorine is 17. Performance & security by Cloudflare, Please complete the security check to access. Add your answer and earn points. You may need to download version 2.0 now from the Chrome Web Store. A chloride ion has 18 electrons total. This makes chlorine's valence shell incomplete, and the atom attracts 'loose' electrons to this shell to complete it, so the atom can be stable.
When chlorine forms a chloride ion, an atom accepts an electron and becomes Cl- (negative ion).
Cl Number Of Valence Electrons
Cloudflare Ray ID: 5ec3774dff69e233 Fact Check: What Power Does the President Really Have Over State Governors? By strict definition, most transitional metals have two valence electrons, but may have a larger range of apparent valence electrons. Information about your device and internet connection, including your IP address, Browsing and search activity while using Verizon Media websites and apps. Since elements in the same family have the same number of valence electrons, their dot structures will look the same, too! • Use iron as an example, a transitional metal with the symbol Fe, atomic number 26 , located at period 4, group 8. Cl2 is the diatomic molecule of chlorine (Cl) . How Many Electrons Does a Chloride Ion Have. temiloluwaaaaa is waiting for your help. ), The Secret Science of Solving Crossword Puzzles, Racist Phrases to Remove From Your Mental Lexicon. Festival of Sacrifice: The Past and Present of the Islamic Holiday of Eid al-Adha. •
Will 5G Impact Our Cell Phone Plans (or Our Health?! Each shell after that initial shell holds up to eight electrons, and each atom has rings to accommodate all the electrons, but no more.
Chlorine has 17 electrons; therefore, the first ring is filled with two electrons, the second ring is filled with eight electrons, but there are only seven electrons for the third ring. B) Find the acceleration of the c … ar rolling down the hill.
Locate the transition metal on the periodic table and make note of the group number. The chloride ion has an added electron to complete the outermost electron shell, or valence shell, of the atom. Another way to prevent getting this page in the future is to use Privacy Pass. Your IP: 188.166.242.150
This added electron gives the chloride ion a negative charge and is thus considered an anion. Completing the CAPTCHA proves you are a human and gives you temporary access to the web property. You can change your choices at any time by visiting Your Privacy Controls. The first shell, closest to the atom, holds two electrons. As for the symbol, remember that an atom wants 8 electrons in its outer ring if possible. We and our partners will store and/or access information on your device through the use of cookies and similar technologies, to display personalised ads and content, for ad and content measurement, audience insights and product development. Find out more about how we use your information in our Privacy Policy and Cookie Policy. How many valence electrons does chlorine have? Cloride is a chlorine atom, which has found that last electron and added it to the outermost valence shell to become stable. Chlorine is a highly reactive element, due to the outermost shell of electrons. A car weighing 12,000 N is parked on a 36° slope. If you are at an office or shared network, you can ask the network administrator to run a scan across the network looking for misconfigured or infected devices.
Chlorine has 17 electrons; therefore, the first ring is filled with two electrons, the second ring is filled with eight electrons, but there are only seven electrons for the third ring. A) Find the force tending to cause the car to roll down the hill.
This is the halogen group, and all of them have 7 valence electrons (hence the column: 7). ION: an atom that has gained or lost one or more electrons **atoms with the same atomic number (protons), but different number of electrons §An atom that has LOST one or more electrons (has an overall positivecharge) 11 Protons 8 Neutrons 19Na 11. It has 14 valence electrons, 7 in each atom. Electrons fill rings, known as shells, outside the atom. Is the Coronavirus Crisis Increasing America's Drug Overdoses? To enable Verizon Media and our partners to process your personal data select 'I agree', or select 'Manage settings' for more information and to manage your choices. Yahoo is part of Verizon Media. Chlorine is in column 7 (immediately before the noble gases). ••• raeva/iStock/Getty Images. Valence Electrons Graph - Valence Electrons of all the elements in graph.
List Of Valence Electrons For Each Element
Stuntman Salary Uk,Usc Research Studies,Yoshino Cherry Tree Leaves,Mark Nicholas Family,Nikolay Storonsky Wife,Marshall Society Essay Competition,Adam Lz Car Collection List,Chi With Ac Merch,Meltdown Gta 5,Pagan Savior Persona 5 Royal,Remixed Pixel Dungeon Blessing,Percy Jackson Is Born A Son Of Chaos Fanfiction,Paige Winter Now,Zaz 965 For Sale,How To Make Lightweight Shovel In Escapists,Research Paper Topics For Landscape Architecture,Charlie Parker Woai Age,Sara Molina And Tekashi69,Zeus Familia Danmachi,Liebherr Red Bull Fridge,Warthog Forza Horizon 4,Kanakuk Packing List,Mastermix Warm Up Mixes,Renee Rapp Height,Canpar Tracking Cannot Locate,The Handmaid's Tale Isolation Quotes,Arabic Swear Words In Arabic,Wally Lewis Net Worth,Skyrim Requiem Wiki,Cool Roblox Names For Boys,Homeschool Reimbursement Michigan,Cougar Wild Cats Alabama,Amazon Fire Tv Stuck At Bootscreen,What Musical Is Poisoning Pigeons In The Park From,Ingles Product Search,Dbd Demogorgon Build,Ultra Classic For Sale Craigslist,Who Is The Girl In Midland Mr Lonely Video,Wild Oscar Fish For Sale,Regulus Black Self Harm Fanfiction,1930s Bungalow Uk,Multi Bet Of The Day,How Does Domino's Contactless Carryout Work,Middle Name For Winnie,Havanese Weight Calculator,Jasleen Name Meaning In Punjabi,Ube Coffee Cake,Sergio Aragones Domenech Obama,Sir Dinshaw Maneckji Petit, 5th Baronet (born 1965),Bower Vine Yellow Leaves,Suture Kit With Lidocaine Amazon,Kate Walsh Sister,Megaman X8 Booster Forest Items,Republic Flooring Reviews,Michael Imperioli Kids,Is Disquete Masculine Or Feminine In French,Angry Kid Meme Red Shirt,Christopher Estevez Rbc,Night Bus Samuel Hopkins Adams Pdf,安倍 プーチン 身長,Nicole Johnson Height,Carpet Glue Remover Machine Rental,Elias Koteas De Niro,Caerphilly Cheese Substitute,Corey White Aboriginal,Harwood Arms Dallas,
Cl Number Of Valence Electrons
Leave a comment
Cl Element Valence Electrons
You must be logged in to post a comment.
